Natural Medicine Journal
Article Summary
Osteoarthritis, the most common form of arthritis, results primarily from a progressive degeneration of cartilage glycosaminoglycans (GAGs). Standard drug therapy suppresses pain and inflammation, but actually promotes progression of the disease process by inhibiting GAG synthesis and cartilage repair. In contrast, glucosamine sulfate offers an effective treatment for osteoarthritis by providing the rate-limiting step in GAG synthesis. Glucosamine serves as the fundamental building block for GAGs. Numerous double-blind studies have shown glucosamine sulfate to produce better results than standard drug therapy. The pharmacology and clinical features of glucosamine sulfate are reviewed.
Osteoarthritis, also known as degenerative joint disease, is the most common form of arthritis. It may be the most prevalent disease in America. Surveys have indicated that over 40 million Americans have osteoarthritis. It is seen primarily, but not exclusively, in the elderly.
The Weight-bearing joints, like the knees and hips, and joints of the hands are the joints most often affected with osteoarthritis. In affected joints, there is much cartilage destruction followed by hardening and the formation of large bone spurs in the joint margins. Pain, deformity, and limitation of motion in the joint results.
The onset of osteoarthritis can be very subtle, morning joint stiffness is often the first symptom. As the disease progresses, there is pain on motion of the involved joint that is made worse by prolonged activity and relieved by rest.
What Causes Osteoarthritis?
The cumulative effects of decades of use leads to degenerative changes in joints. This damage is compounded by a decreased ability to repair joint structures. Specifically, with aging, there is a decreased ability to restore and manufacture normal joint structures like cartilage. Much of this reduced function may reflect nutritional status.
A broad-range of nutrients have been shown to be critical to healthy joints. A deficiency of any of these nutrients can result in impaired cartilage structure or function.
Arthritis Medications
Clinical and experimental research indicates that current drugs being used in osteoarthritis may be producing short-term benefit, but actually accelerating the progression of the joint destruction.
The first drug generally used in the treatment of osteoarthritis is aspirin. It is often quite effective in relieving both the pain and inflammation. It is also fairly inexpensive. However, since the therapeutic dose required is relatively high (2 to 4 grams per day), toxicity often occurs. Tinnitus (ringing in the ears) and gastric irritation are early manifestations of toxicity.
Other nonsteroidal anti-inflammatory drugs (NSAIDs) are often used, especially when aspirin is ineffective or intolerable. The following are representative of this class of drugs; ibuprofen (Motrin), fenoprofen (Nalfon), indomethacin (Indocin), naproxen (Naprosyn), tolmetin (Tolectin), and sulindac (Clinoril). These drugs are also associated with side effects including gastrointestinal upset, headaches, dizziness, and are therefore recommended for only short periods of time.
One side effect of aspirin and other NSAIDs that is often not mentioned is their inhibition of cartilage repair and acceleration of cartilage destruction.1-3 Because osteoarthritis is caused by a degeneration of cartilage, it appears that while NSAIDs are fairly effective in suppressing the symptoms, they possibly worsen the condition by inhibiting cartilage formation and accelerating cartilage destruction. This has been upheld in clinical studies which have shown that NSAIDs use is associated with acceleration of osteoarthritis and increased joint destruction.4-6 Simply stated, aspirin and other NSAIDs appear to suppress the symptoms but accelerate the progression of osteoarthritis. Their use should be avoided.
Natural alternative to arthritis medications
If current arthritis medications should be avoided, what is an arthritis sufferer to do? A naturally occurring substance found in high concentrations in joint structures appears to be nature’s best remedy for osteoarthritis. This compound is glucosamine sulfate.
This simple molecule is composed of glucose, an amine (nitrogen and two molecules of hydrogen), and sulfur. The manufacture of glucosamine is the rate-limiting step in GAG synthesis. Glucosamine is formed from the glycolytic intermediate fructose-6-phosphate via amination with glutamine acting as the donor, yielding glucosamine-6-phosphate which is then acetylated and/or converted to galactosamine for incorporation into the growing GAG.
The main physiological function of glucosamine on joints is to stimulate the manufacture of cartilage components as well as promote the incorporation of sulfur into cartilage. In other words, glucosamine is not only responsible for stimulating the manufacture of substances necessary for proper joint function, it also is responsible for stimulating joint repair.
It appears that as some people age, they lose the ability to manufacture sufficient levels of glucosamine. The result is that cartilage loses its ability to act as a shock absorber. The inability to manufacture glucosamine has been suggested to be the major factor leading to osteoarthritis. This link lead researchers in Europe to ask an important question, “What would happen if individuals with osteoarthritis took glucosamine?” The results have been astonishing.
Clinical Trials
Numerous double-blind studies have shown glucosamine sulfate to produce much better results compared to NSAIDs and placebos in relieving the pain and inflammation associated with osteoarthritis. This is despite the fact that glucosamine sulfate exhibits very little direct anti-inflammatory effect and no direct analgesic or pain relieving effects.7-13
While NSAIDs offer purely symptomatic relief and may actually promote the disease process, glucosamine sulfate appears to address the cause of osteoarthritis. By getting at the root of the problem, glucosamine sulfate not only improves the symptoms including pain, it also helps the body repair damaged joints. This effect is outstanding, especially when glucosamine’s safety and lack of side effects is considered.
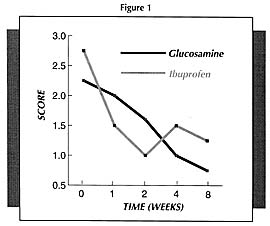
It must be pointed out that the beneficial results with glucosamine are more obvious the longer it is used. Because glucosamine sulfate is not an anti-inflammatory or pain relieving drug per se, it takes a while longer to produce results. But once it starts working, it will produce much better results compared to NSAIDs.
For example, in one study (Figure 1) which compared glucosamine sulfate to ibuprofen (the active ingredient of Motrin, Advil, and Nuprin), pain scores decreased faster in the first 2 weeks in the ibuprofen group; however, by week 4, the group receiving the glucosamine sulfate was doing significantly better than the ibuprofen group.11 Physicians rating the overall response as good or fair rated 44% of the glucosamine sulfate-treated patients as good compared to only 15% of the ibuprofen group.
Results from a large, open trial
In addition to showing benefit in double-blind studies, oral glucosamine sulfate was shown to offer significant benefit in an open trial involving 252 doctors and 1,506 patients in Portugal.14 This large study provides valuable clinical information on the appropriate use of glucosamine sulfate.
The patients in this study received 500 mg of glucosamine sulfate three times daily over a mean period of 50+/-14 days. The results were analyzed and showed that the symptoms of pain at rest, on standing, and on exercise and limited active and passive movements improved steadily throughout the treatment period.
Objective therapeutic efficacy was rated by doctors as “good” in 59% of patients, and “sufficient” in a further 36%. Therefore, a total of 95% of patients achieved benefit from glucosamine sulfate. The results with glucosamine sulfate were rated by both doctors and patients as being significantly better than those obtained with previous treatment including NSAIDs, vitamin therapy, and cartilage extracts.
Only injectable glucosamine sulfate was comparable to the oral glucosamine, but even that was less effective. Glucosamine sulfate produced good benefit in a significant portion of patients who had not responded to any other medical treatment.
Complete tolerability of oral glucosamine was reported by a significantly larger proportion of patients than with any other treatment. Possible adverse reactions occurred in only 12.1% of patients. All of these possible side effects were light to moderate gastrointestinal symptoms including epigastric pain or tenderness, heartburn, diarrhea, nausea, and dyspepsia.
Obesity is associated with a significant shift from good to fair. This finding may indicate that higher dosages may be required for obese individuals or that oral glucosamine is not enough to counteract the stress of obesity on the joints.
associated with a shift from good to sufficient in efficacy, as well as tolerance. Individuals with current peptic ulcers should try and take glucosamine sulfate with foods. Individuals taking diuretics may need to increase the dosage to compensate for the reduced effectiveness.
The improvement with glucosamine lasted for a period of 6 to 12 weeks after the end of treatment. This result indicates that a repeated course of administration are necessary. Given the safety and excellent tolerability of glucosamine, it is suitable for long-term use, even if continuous.
Glucosamine sulfate vs. cartilage extracts
Cartilage extracts, including purified chondroitin sulfate, sea cucumber, green-lipped mussel, and shark cartilage, are popular nutritional supplements which may also help osteoarthritis by improving cartilage function. However, these compounds differ in their degree of purity and effectiveness in osteoarthritis compared to glucosamine sulfate.
Shark cartilage, sea cucumber, and green-lipped mussel contain a mixture of GAGs. One of the key GAGs is chondroitin sulfate. Chondroitin sulfate is composed of repeating units of glucosamine with attached sugar molecules.
The difference between glucosamine sulfate, cartilage extracts, and chondroitin sulfate products is similar to the difference between crude ore (shark cartilage or chondroitin sulfate) and pure gold (glucosamine). While there is gold in crude ore, if you are trying to make jewelry, it is better to use the pure gold. If you are trying to restore cartilage and joint structures, it is best to use glucosamine sulfate rather than chondroitin sulfate or shark cartilage.
The key reason is the improved absorption and utilization of glucosamine sulfate. Cartilage extracts, shark cartilage, green-lipped mussel, sea cucumber, and chondroitin sulfate products are composed of large molecules that are extremely difficult to absorb. The absorption rate for chondroitin sulfate, the smallest molecule in these products, is estimated to be between zero and 8%.15 In contrast, detailed pharmacokinetic studies in animals and humans have shown up to 98% of orally administered glucosamine sulfate is absorbed.16,17
These pharmacokinetic studies have shown that after glucosamine sulfate is absorbed, it is preferentially taken up by cartilage and other joint structures, where it then simulates the manufacture of chondroitin sulfate and other mucopolysaccharides. One of its key effects is to also stimulate the incorporation of sulfur into cartilage.
While the effectiveness of oral glucosamine sulfate has much documentation, the effectiveness of oral cartilage extracts, chondroitin sulfate, green-lipped mussel, sea cucumber, and shark cartilage in osteoarthritis is a subject of much debate. The positive clinical studies with glycosaminoglycan preparations have utilized injectable forms.18-20 The use of pharmaceutical grade cartilage preparations and chondroitin sulfate injections, according to established protocols has well-documented benefit, but the benefits are less than that attributed to glucosamine sulfate.
When all is considered, it is quite easy to see why glucosamine sulfate is preferred to cartilage extracts in the treatment of osteoarthritis.
Glucosamine sulfate vs. NAG
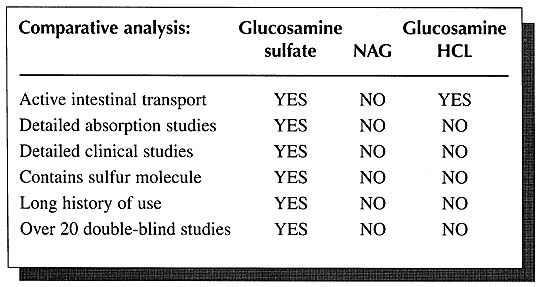
Currently companies marketing N-acetyl-glucosamine, commonly referred to as “NAG,” are misleading many physicians into believing that NAG is better absorbed, more stable, and is better utilized than glucosamine sulfate. These contentions are without support in the scientific literature. In fact, the literature contains just the opposite. Glucosamine sulfate is clearly the preferred form.
As mentioned above, detailed human studies on the absorption, distribution, and elimination of orally administered glucosamine sulfate have shown an absorption rate of as high as 98% and that once absorbed it is then distributed primarily to joint tissues where it is incorporated into the connective tissue matrix of cartilage, ligaments, and tendons, In addition, there are the impressive clinical studies on thousands of patients. In contrast, there has never been a double-blind study using NAG for any application. Nor have there ever been any detailed absorption studies on NAG in humans.
Further evidence of the superiority of glucosamine sulfate to NAG is offered by studies in laboratory animals. Over the years, numerous researchers have researchers have repeatedly demonstrated that glucosamine is superior to NAG in terms of absorption and utilization by at least a factor of 2:1.18-29 These researchers have concluded that glucosamine is a more efficient precursor of macromolecular hexosamine [glycosaminoglycans] than N-acetyl-glucosamine does not penetrate the cell membranes and, as a result, is not available for incorporation into glycoproteins and mucopolysaccharides.20
The absorption of NAG is quickly digested by intestinal bacteria; 2) NAG is a known binder of dietary lectins in the gut with the resultant lectin-NAG complex being excreted in the feces; and 3) a large percentage of NAG is broken down by intestinal cells.
NAG differs from glucosamine sulfate in that instead of a sulfur molecule, NAG has a portion of an acetic acid molecule attached to it. Glucosamine sulfate and NAG ware entirely different molecules and appear to be handled by the body differently. The body preferentially utilizes glucosamine sulfate compared to NAG. This preference is exhibited by the fact that the absorption of glucosamine sulfate is an active process.29 In other words, there are mechanisms in the body which are designed specifically for the absorption and utilization of glucosamine sulfate. No such mechanisms exist for NAG.
It is highly unlikely that NAG possesses the same kind of antiarthritic and antireactive properties that glucosamine sulfate has been shown to possess.30-31 In addition to the question of absorption, several studies have shown that the articular tissue is not able to utilize NAG as well as it does glucosamine.18-19
The marketing information on NAG will often use the term slow acetylators to describe a very small group of individuals with Crohn’s disease and ulcerative colitis who are unable to convert glucosamine to NAG as fast as individuals without these diseases. Glucosamine and NAG are necessary in the manufacture of mucin, the glycoprotein lining of the intestinal tract.
Distributors of NAG hold up only one study as evidence that NAG is better. The study demonstrated that when intestinal cells from patients with Crohn’s disease or ulcerative colitis were bathed in a solution containing a ratio of radioactive NAG:glucosamine of 10:1, the cells incorporated more NAG than the cells from individuals without these diseases.30 These results are expected due to the higher concentrations of NAG in the media artificially promoting passive diffusion to a greater extent than the active accumulation of glucosamine. How distributors of NAG can then use this information to claim that NAG is better than glucosamine sulfate is puzzling since the significance of this test tube study is unclear and other studies have demonstrated an increased utilization of glucosamine in these patients.33
The problem of acetylation of glucosamine is not a factor for most people as it is not a rate-limiting step in the manufacture of glycosaminoglycans, instead it is the manufacture of glucosamine.
Another form of glucosamine presently being marketed is glucosamine hydrochloride (HCI). As with NAG, the research simply does not support the use of glucosamine HCI.
It appears the sulfur component of glucosamine sulfate may be critical to the beneficial effects noted. Sulfur is an essential nutrient for joint tissue where it functions in the stabilization of the connective tissue matrix of cartilage, tendons, and ligaments. As far back as the 1930’s, researchers demonstrated that individuals with arthritis are commonly deficient in this essential nutrient.34 Restoring sulfur levels brought about significant benefit to these patients.35 Therefore, it appears the sulfur portion of glucosamine sulfate is extremely important and is another reason why glucosamine sulfate is the preferred form of glucosamine.
Dosage Information
The standard dose for glucosamine sulfate is 500 mg three times per day. Obese individuals may need higher dosages based on their body weight (20 mg/kg body weight/day).
Glucosamine sulfate is extremely well-tolerated. In addition, there are no contra-indications or adverse interactions with drugs. Individuals taking diuretics may need to take higher dosages. Glucosamine sulfate may cause some gastrointestinal upset (nausea, heartburn, etc.) in rare instances. If this occurs, have the patient try taking it with meals.
References
- Brandt KD: Effects of nonsteroidal anti-inflammatory drugs on chondrocyte metabolism in vitro and in vivo. Am J Med 83(suppl.5A):29-34, 1987.
- Shield MJ: Anti-inflammatory drugs and their effects on cartilage synthesis and renal function. Eur J Rheumatol Inflam 13:7-16, 1993.
- Brooks PM, Potter Sr and Buchanan WW;NSAID and osteoarthritis – help or hindrance. J Rheumatol 9:3-5, 1982.
- Newman, N.M. and Ling, R.S.M. Acetabular bone destruction related to non-steroidal anti-inflammatory drugs. Lancet; ii; 11-13, 1985.
- Solomon L; Drug induced arthropathy and necrosis of the femoral head. J Bone Joint Surg 55B:246-51, 1973.
- Ronnigen H and Langeland N; Indomethacin treatment in osteoarthritis of the hip joint. Acta Orthop Scand 50; 169-74, 1979.
- Crolle G and D’este E; Glucosamine sulfate for the management of arthorosis; a controlled clinical investigation. Curr Med Res Opin 7;105-9, 1980.
- Pujalte JM, et al.; Double-blind clinical evaluation of oral glucosamine sulphate in the basic treatment of osteoarthrosis. Curr Med Res Opin 7;110-4, 1980.
- Drovanti A, et al.; Therapeutic activity of oral glucosamine sulfate in osteoarthrosis; a placebo-controlled double-blind investigation. Clin Ther 3;260-72, 1980.
- Vajaradul Y; Double-blind clinical evaluation of intra-articular glucosamine in outpatients with gonarthosis. Clin Ther 3;336-43, 1981.
- Vaz AL; Double-blind clinical evaluation of the relative efficacy of ibuprofen and glucosamine sulfate in the management of osteoarthrosis of the knee in out-patients. Curr Med Res Opin 8;145-9, 1982.
- D’Ambrosia ED et al.; Glucosamine sulphate; a controlled clinical investigation in arthrosis. Pharmatherapeutica 2;504-8, 1982.
- Reichelt A, et al.; Efficacy and safety of intramuscular glucosamine sulfate in osteoarthritis of the knee. A randomized, placebo-controlled, double-blind study. Arzniem Forsch 44;75-80, 1994.
- Tapadinhas MJ, et al.; Oral glucosamine sulfate in the management of arthrosis; report on a multi-centre open investigation in Portugal. Pharmatherapeutica 3;157-68, 1982.
- Morrison M; Therapeutic applications of chondroitin-4-sulfate, appraisal of biologic properties. Folia Angiol 25;225-32, 1977.
- Setnikar I, et al.; Pharmacokinetics of glucosamine in man. Arzneim Forsch 43(10);1109-13, 1993.
- Setnikar I, et al.; Pharmacokinetics of glucosamine in the dog and man. Arzneim Forsch 36(4);729-35, 1986.
- Karzel K and Domenjoz R; Effect of hexosamine derivatives and uronic acid derivatives on glycosaminoglycan metabolism of fibroblast cultures. Pharmacology 5;337-45, 1971.
- Vidal y Plana PR, et al.; Articular cartilage pharmacology; I. In vitro studies on glucosamine and non steroidal anti-inflammatory drugs. Pharmacol Res Comm 10;557-69, 1978.
- Capps JC, et al.; Hexosamine metabolism. II. Effect of insulin and phlorizin on the absorption and metabolism, in vivo, of D-glucosamine and N-acetyl-glucosamine in the rat. Biochim Biophys Acta 127;205-12, 1966.
- Capps JC, et al.; Hexosamine metabolism. I. The absorption and metabolism, in vivo of orally administered D-glucosamine and N-aecetyl-D-glucosamine in the rat. Biochim Biophys Acta 127;194-204, 1966.
- Shetlar MR, et al.; Incorporation of radioactive glucosamine into the serum proteins of intact rats and rabbits. Biochim Biophysica Acta 83;93-101, 1964.
- Richmond JE; Studies on the metabolism of plasma glycoproteins. Biochemistry 2(4);676-83-101, 1964.
- Capps JC and Shetlar MR; In vivo incorporation of D-glucosamine-1-C14 into acid mucopolysachharides of rabbit liver. Proc Soc Exptl Biol Med 114;118-20, 1963.
- Shetlar MR, et al.; Fate of radioactive glucosamine administered parenterally to the rat. Proc Soc Exptl Biol Med 109;335-7, 1962.
- Kohn P, et al.; Metabolism of D-glucosamine and N-acetyl-D-glucosamine in the intact rat. J Biol Chem 237(2);304-8, 1962.
- McGarrahan JF and Maley F; Hexosamine metabolism. J Biol Chem 237(8);2458-65, 1962.
- Shetlar MR, et al.; Incorporation of [1-14C]glucosamine into serum proteins. Biochim Biophys Acta 53:615-6, 1961.
- Tesoriere G, et al.; Intestinal absorption of glucosamine and N-acetylglucosamine. Experientia 28;770-1, 1972.
- Setnikar I, et al.; Antiarthritic effects of glucosamine sulfate studied in animal models. Arzneim-Forsch 41;542-5, 1991.
- Setnikar I, et al.; Antireactive properties of glucosamine sulfate. Arzneim Forsch 41(2);157-61, 1991.
- Burton AF and Anderson FH; Decreased incorporation of 14C-glucosamine relative to 3H-N-acetylglucosamine in the intestinal mucosa of patients with inflammatory bowel disease. Am J Gastroenterol 78;19-22, 1983.
- McDermott RP, et al.; Glycoprotein synthesis and secretion by mucosal biopsies of rabbit colon and human rectum. J Clin Invest 54;545-54, 1974.
- Sullivan MX and Hess WC; Cystine content of finer nails in arthritis. J Bone Joint Surg 16;185-8, 1935.
- Senturia BD; Results of treatment of chronic arthritis and rheumatoid conditions with colloidal sulphur. J Bone Joint Surg 16;119-25, 1934.

